Quality procedures according to the ISO 9001 Standard requirements – examples, samples, and solutions
What is the meaning of quality procedures? What is so qualitative about them? Ah! A key question. These quality procedures are the heart and soul of your quality management system. These procedures establish processes that make sure everything is performed according to the ISO 9001 Standard requirements.But first, let’s review shortly and understand what a procedure is.
Important notice – the ISO 9001:2015 does not require the maintenance of quality procedures anymore and the quality manual anymore. It does not mean that you will have to dispose of them – they can still be used in you QMS. I recommend to keep them but adapt them the new requirements of the ISO 9001:2015, for example no need for a procedure for preventive action anymore.
ISO 9001 – Working Procedures
One of the most important principles of the ISO 9001 Standard applies simply: “say what you do, do what you say”
That actually means that you must define first what you are required to perform and then perform it! One can also explain it differently; you will define in which cage (procedures and procedures hence requirements) you want to spend your time and work; the working processes and working instructions hence requirements. The purpose of the working instructions or procedures is to explain, very simply, what is required to do be done by employees in their everyday tasks. The instructions are highly important! Why? Who asked that? You are not serious! The explanation is very simple; When you define a working procedure you define a frame. This frame defines what has to be done and leave much less possibilities for questions, nonconformities and faults. Your employees need guidance. Naturally people would try to break the frame they are living and working in, in order to maintain more comfort for themselves or to promote interests that are no conformed to the organization’s objectives. This comes on behalf of efficiency and effectiveness. And when efficiency and effectiveness are declining, you can be pretty much sure that, in the not so long term, profitability and quality would decline as well.
Procedures give you the ability to examine where your employee tries short cuts. Straying from the procedures will create nonconformities. Nonconformities harm your profitability, even if you cannot realize it in the short term. In this case when you define a procedure, it is highly important to define the appropriate control over it as well. This issue is enforced by the ISO 9001:2015 Standard almost fanatically. In fact the requirement 4.4 Quality management system and its processes demands the establishment and maintenance of a method for planning, defining and documenting processes in the QMS. One of the means to eliminate nonconformities and defects is a clear procedure. A procedure must include:
- What is the purpose of the process? The objective or goal of the process.
- Who is responsible for maintaining and performing the process? In order for you to know exactly who is responsible for what had been done.
- What is the method? You must specify the steps, phases, or actions required to perform the process. We recommend being generous with details, specify within the most specific level and to explain with the simplest language what is to be done.
- What are the tools one needs to perform the process? Forms, software, working tools, etc. – This is actually the documentation and control over the procedure. By examining this tools we can decide whether the procedure was maintained and how well.
- What are the process outputs? The outputs expected at the end of the process (a price quote documented, a certificate of calibration, records of any kind, any form, etc).
ISO 9001 – Quality Procedures
After reviewing the meaning and importance of a procedure and understanding the link between a procedure and a process allow me to discuss to the quality procedures. The goal of these procedures is to make sure that the organization performs the minimum requirements of the ISO 9001 Standard that suit all kinds of organizations – from the low tech to the high-tech companies. These procedures are referred to as “quality procedures”. The procedures include the next:
- A quality manual. A document defining the scope of the quality management system, a general presentation of the organization activities, its organizational structure, the context of the organization, the needs and expectations of interested parties, a general description of the main process, a description of the relation between the working process and the quality management system (as shown below), a list of all procedures in the organization and list of exclusions –the ISO 9001 Standard requirements which are not applicable to the organization’s activity or nature. The quality manual is in fact not a procedure but a manual; it must not have the characteristics mentioned above. It describes no activities at all but it is required to maintain it documented as specified. As far for the description of the relation between the working process and the quality management system, I drew this diagram for example:
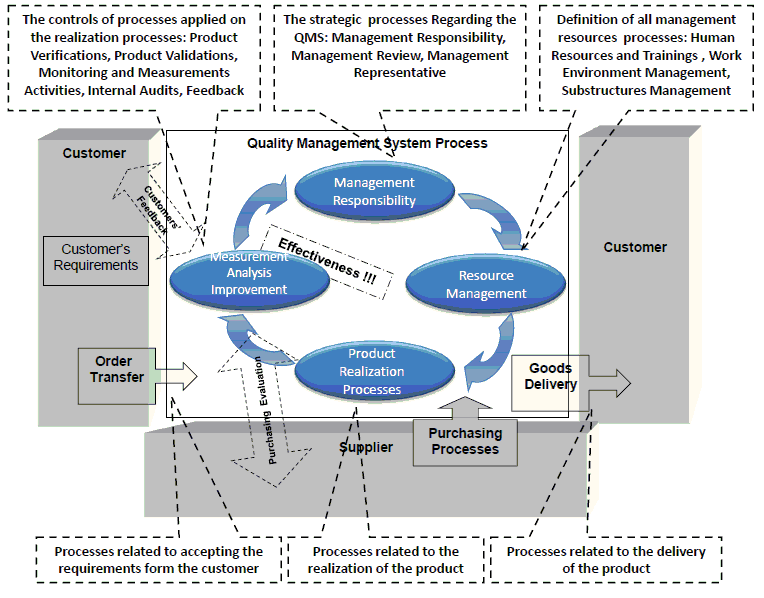
ISO 9001 quality procedures – the quality management relation diagram
- Control of documents. Specification of the process of controlling documents that are included under the quality management system. The requirements are specified in these articles. In the procedure you must define how to achieve the requirements
- Control of records. Specification of the process of controlling your records that are included under the quality management system. The requirements are specified in this article. In the procedure you must define how to achieve the requirements. This procedure will include or refer to a list of all documentation included in the quality management
- Internal audits. A procedure specifying how the internal audit should be performed within the organization. You may refer to this article for the specific requirements. In the procedure you are required to define how to achieve
- Control of nonconformity. Procedure specifying how one should handle nonconformity when he detects it
- Corrective action. A procedure specifying how one implements a corrective action. Read this article to learn about the ISO 9001 Standard requirements for corrective action
This is the list of the required quality procedures. They must be documented and maintained. That means that it’s not enough to document the procedure, you must also prove that you follow what you defined with evidence day by day – perform the quality management activities.
Quality procedures according to the ISO 9001 Standard – Summary
- The quality procedures are the heart and soul of your quality management system
- These quality procedures ensure that you maintain a quality management system according to the ISO 9001 Standard
- Say what you do, do what you say – One of the most important principles of the ISO 9001 Standard
- The purpose of a working procedure is to explain what is required to be done in order to reach a specified result or objective.
- A procedure must include: purpose of the process, the responsible party, the method, the tools and process outputs
- The purpose of quality procedures is to ensure that the organization performs the minimum requirements of the ISO 9001 Standard
- Quality procedures include: quality manual, procedure for the control of documents, procedure for the control of records, procedure for the performance of internal audits, procedure for the control of nonconformity, and procedure for the for integrating and controlling corrective action and preventive action.
